
In the production process of wet wipes products, the microbial safety of wet wipes liquid is directly related to the hygienic quality of the product and the safety of users. Whether it is baby wipes, skin care wipes, makeup remover wipes or cleaning wipes, the liquid part of the wet wipes must undergo strict microbial testing to ensure compliance with national and international quality standards.
This article will systematically introduce the main items, testing methods, limit standards and result interpretation of wet wipes liquid microbial testing.

一. Purpose of testing
The purpose of microbial testing of wet wipes liquid is to:
Ensure that there is no pathogenic bacteria contamination in the liquid
Control the total colony count within the safe range
Verify the effectiveness of the formula preservative system
Meet standards such as GB 15979, ISO 11930, USP <61>/<62>, etc.
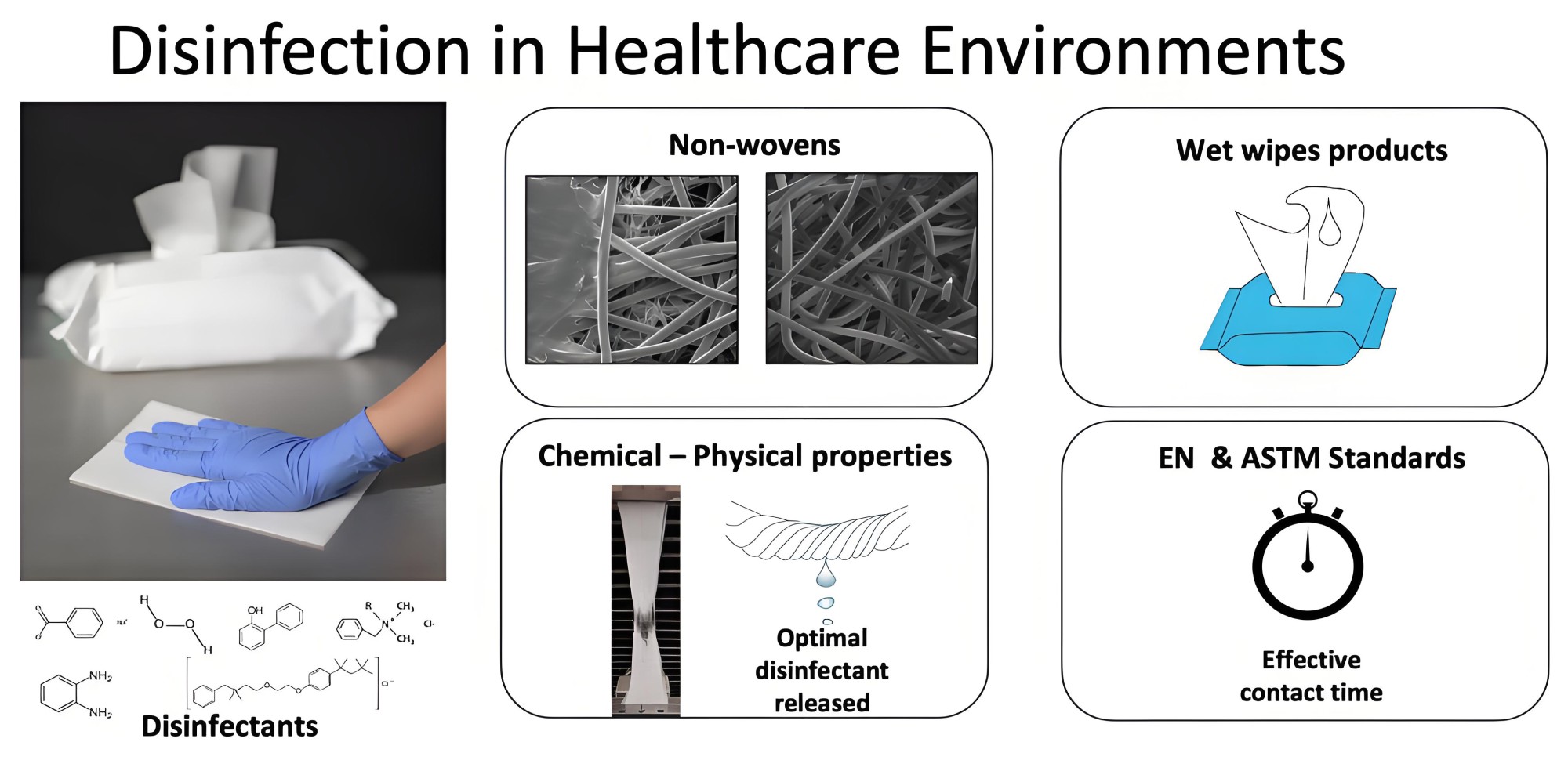
二. Conventional testing items and limits (reference GB 15979)
| Testing items | Limit standard |
| Total colony count (CFU/g or CFU/mL) | ≤1000 |
| Total yeast and mold count | ≤100 |
| Escherichia coli (E. coli) | Not detectable (per 0.1g or 0.1mL) |
| Staphylococcus aureus (S. aureus) | Not detectable |
| Pseudomonas aeruginosa (P. aeruginosa) | Not detectable |
| Candida albicans (C. albicans) | Not detectable (partial type requirement) |
The above standards are mainly applicable to the control requirements of wet wipes liquid as finished products or intermediates before filling or compounding.

三. Detailed explanation of the detection method
1. Total colony count / yeast and mold count
Sample processing:
Take 1g wet wipes or 1mL wet wipes liquid
Add 9mL sterile diluent
Perform 10-fold serial dilution
Culture medium and conditions:
Test items Culture medium Culture conditions
Total colony count NA or TSA 35°C, 48 hours
Total yeast and mold count SDA 25°C, 5-7 days
2. Pathogenic bacteria limit detection (qualitative)
The detection of each pathogen adopts the enrichment-selection separation-confirmation process:
| Microorganism | Enrichment liquid | Selective culture medium | Culture conditions |
| Escherichia coli | Bile salt lactose liquid | EMB or MacConkey | 35°C, 24 hours |
| Staphylococcus aureus | Tryptone water | Baird-Parker agar | 35°C, 48 hours |
| Pseudomonas aeruginosa | Tryptone water | Cetrimide agar | 35°C, 48 hours |
| Candida albicans | SDB | Chromogenic agar | 30°C, 48 hours |
3. Wet wipes liquid preservative challenge test (PET)
This test is used to evaluate the ability of the preservative system in wet wipes liquid to inhibit and kill common microbial contamination, and is widely used in multiple opening products or export-oriented formulas.
Test steps:
Inoculate five representative microorganisms in the wet wipes liquid sample (inoculation volume 10⁵–10⁶ CFU/mL)
Count the remaining microorganisms on days 7, 14, and 28
Compare with ISO 11930 or USP <51> standards to evaluate the preservation effect
Sample data table:
| Microorganism | Initial concentration (log CFU/mL) | Day 7 | Day 14 | Day 28 | Determination |
| Escherichia coli | 6.2 | <1.0 | ND | ND | Pass |
| Staphylococcus aureus | 6.0 | 2.1 | 0.8 | ND | Pass |
| Pseudomonas aeruginosa | 6.1 | <1.0 | ND | ND | Pass |
| Candida albicans | 5.8 | 3.9 | 2.2 | 1.2 | Meets the requirements |
| Aspergillus | 5.5 | 4.8 | 2.5 | 1.6 | Meets the requirements |
Note: ND means not detected, detection limit <1.0 log CFU/mL

四. Testing recommendations and summary
For wet wipes liquid manufacturers, brand owners and OEM companies, microbial control should run through the entire production process, including:
Initial inspection of raw materials and water sources
Process testing of intermediates and final liquids
Batch sample verification of final products
Perform anticorrosion challenge tests regularly (ISO 11930)
A stable and reliable wet wipes liquid microbial testing system is not only a guarantee of product compliance, but also an important endorsement of brand quality.




 English
English
 USA
USA
 西班牙语
西班牙语
 俄罗斯
俄罗斯
 葡萄牙
葡萄牙
 印尼
印尼
 巴基斯坦
巴基斯坦
 尼日利亚
尼日利亚
 孟加拉
孟加拉
 墨西哥
墨西哥
 越南
越南
 日本
日本
 韩国
韩国

