
For wet wipe manufacturers, a successful product relies more than just on the non-woven fabric or packaging design; the stability of the wet wipe liquid is a key factor truly impacting product quality and customer satisfaction.
If the wet wipe liquid is unstable, it may exhibit the following:
Yellowing and discoloration
Odor
Separation and precipitation
Microbial growth and mold
Entire batch returns and customer complaints
To ensure that potential issues with wet wipe liquids are detected and eliminated before use, ensuring the safety of finished wet wipes, five stability tests are essential quality control steps for every wet wipes factory.
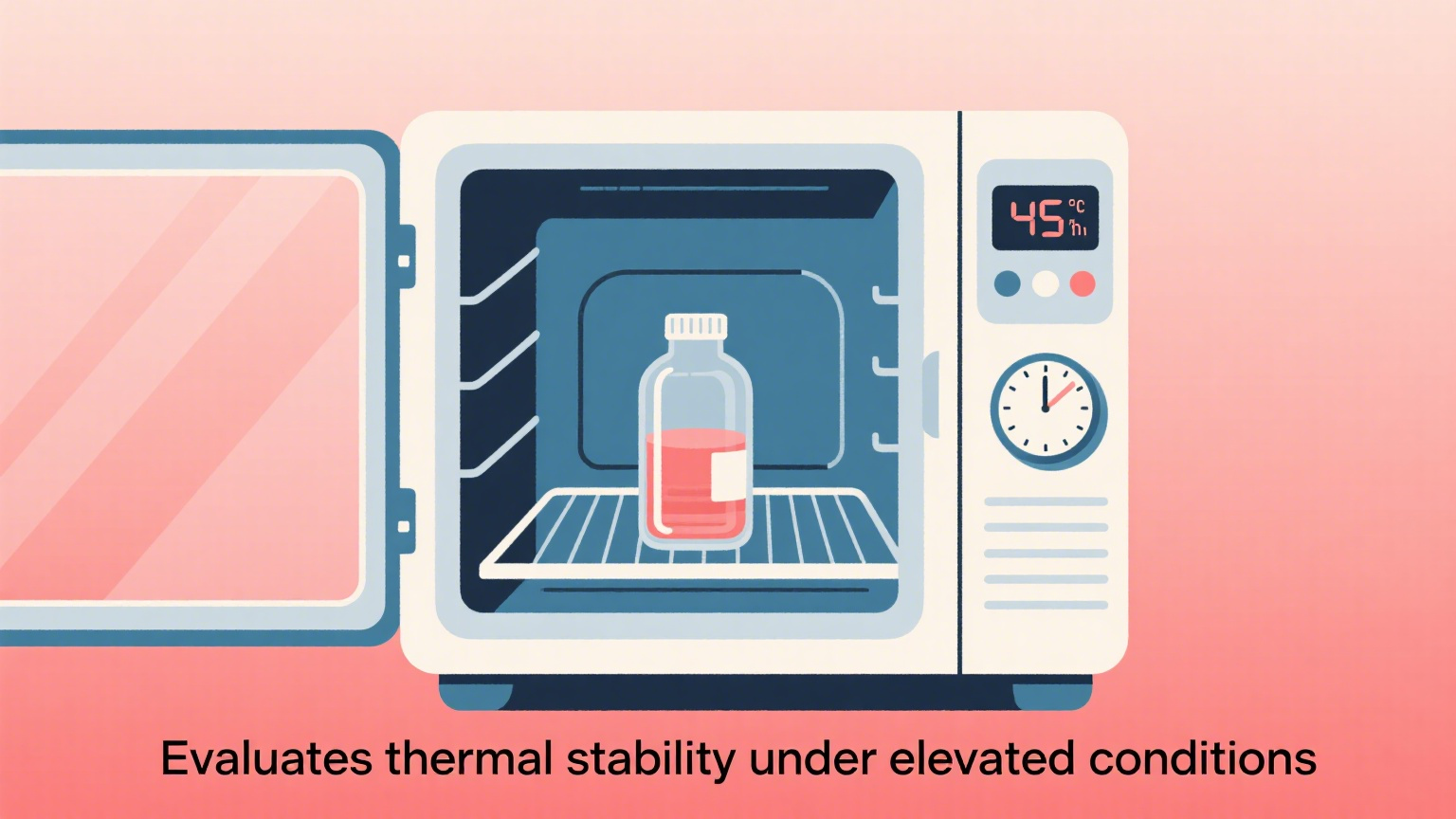
1 High-Temperature Test
Purpose:
Verify the high-temperature resistance of wet wipe liquids and determine whether they deteriorate during high-temperature transportation or storage in summer.
Method:
Place wet wipe liquid samples in incubators at 45°C and 55°C for 7 and 14 days, respectively, and observe:
Discoloration
Presence of precipitation or turbidity
Odor
Reminder:
Formulas containing fragrances, natural plant extracts, vitamins, etc. may be more susceptible to deterioration at high temperatures and should be tested specifically.
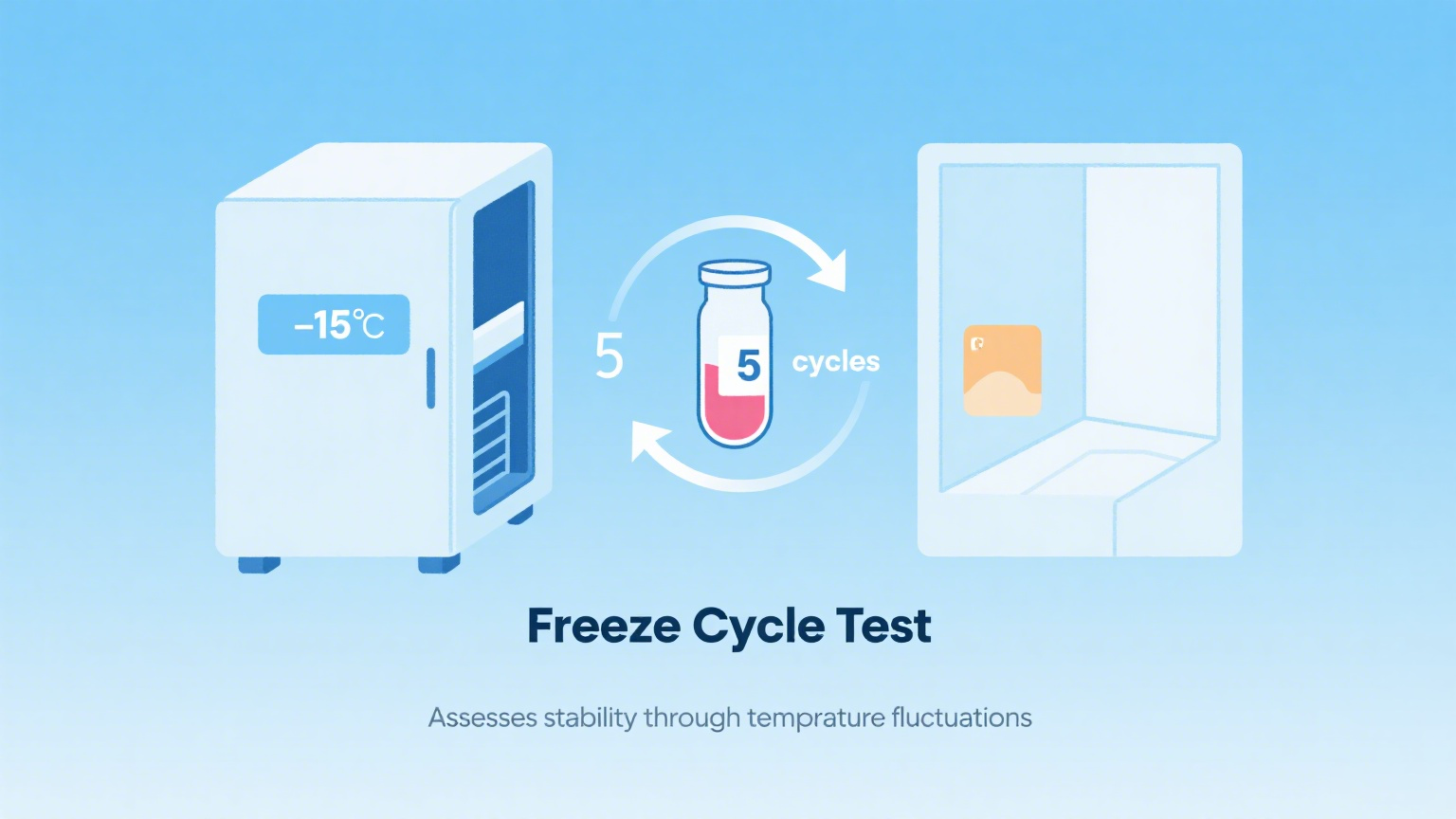
2 Freeze Cycle Test
Purpose:
Simulate low-temperature conditions encountered during winter storage, transportation, and export to cold countries to verify the stability of the wet wipe liquid and determine whether it delaminates or freezes, rendering it unusable. Method:
Place the sample in a freezer at -5°C for more than 24 hours, then return it to room temperature.
Repeat this process 3-5 times.
Observe for:
Liquid separation, precipitation, turbidity, sedimentation, or changes in viscosity.
Recommendation:
This test is recommended for products exported to cold northern regions, such as Europe, the United States, and Russia.

3 pH Stability Test
Purpose:
Develop an appropriate pH for the wet wipe liquid based on its different functions, and ensure that the pH remains stable under various conditions. Only a stable pH ensures the mildness and antiseptic properties of the wet wipe.
Method:
Use a pH meter to test the liquid's pH at room temperature, high temperature, and freezing conditions to ensure it remains within the appropriate range.
✅ Reference Range:
Baby wipes: pH 4.5–6.0
General cleaning wipes: pH 5.0–7.5
Why it matters:
pH fluctuations can weaken preservatives and increase the risk of microbial contamination in the wipes liquid.
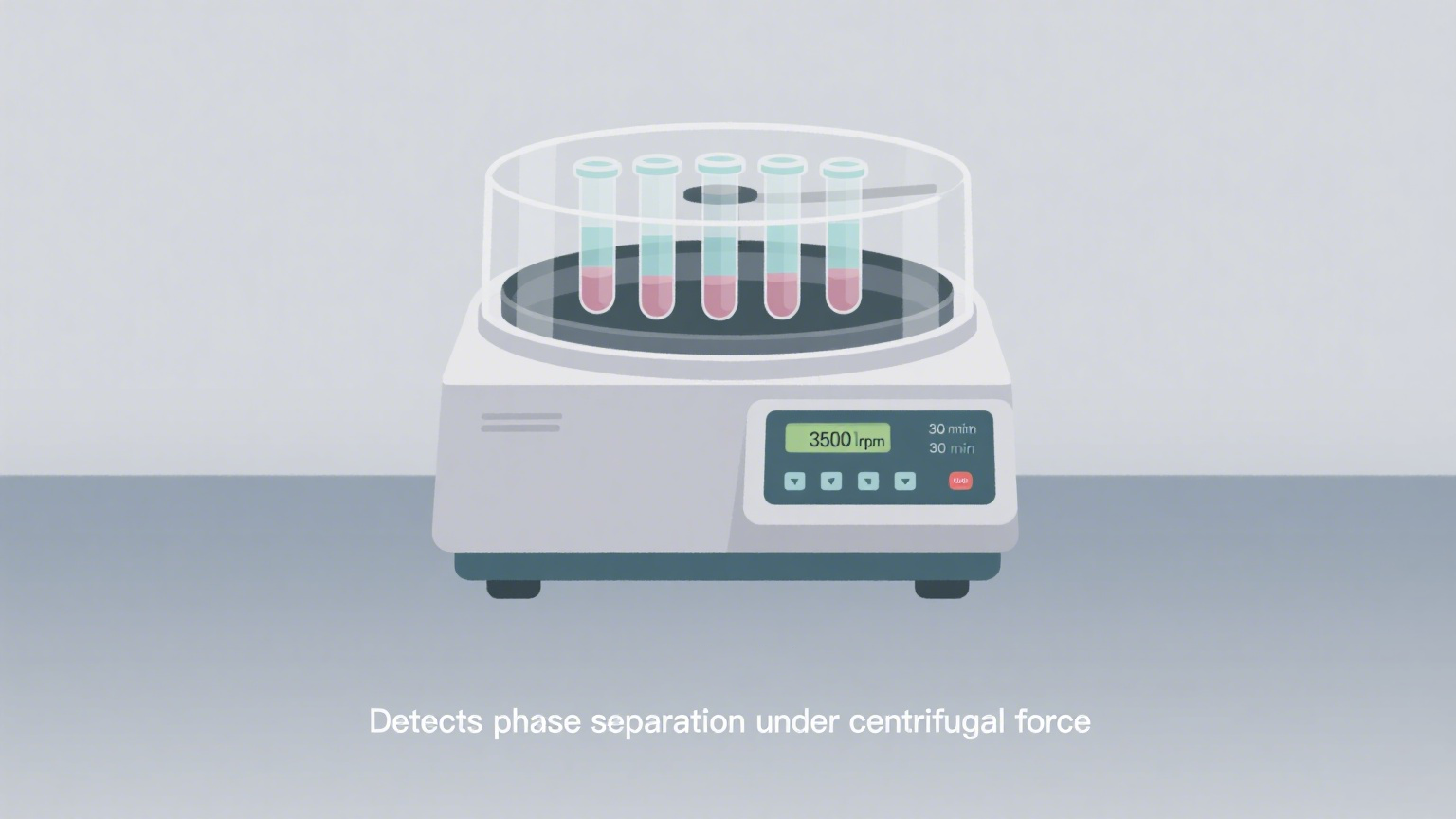
4 Centrifuge Test
Purpose:
To simulate vibration or movement during shipping and test for emulsion or phase stability in the wipes liquid.
Method:
Spin the sample in a centrifuge at 3000 rpm for 30 minutes. Observe for:
Layer separation
Suspended particles
Emulsion breakdown
✅ Especially important for:
Wipes liquid formulas containing oils, emulsifiers, or essential oils.
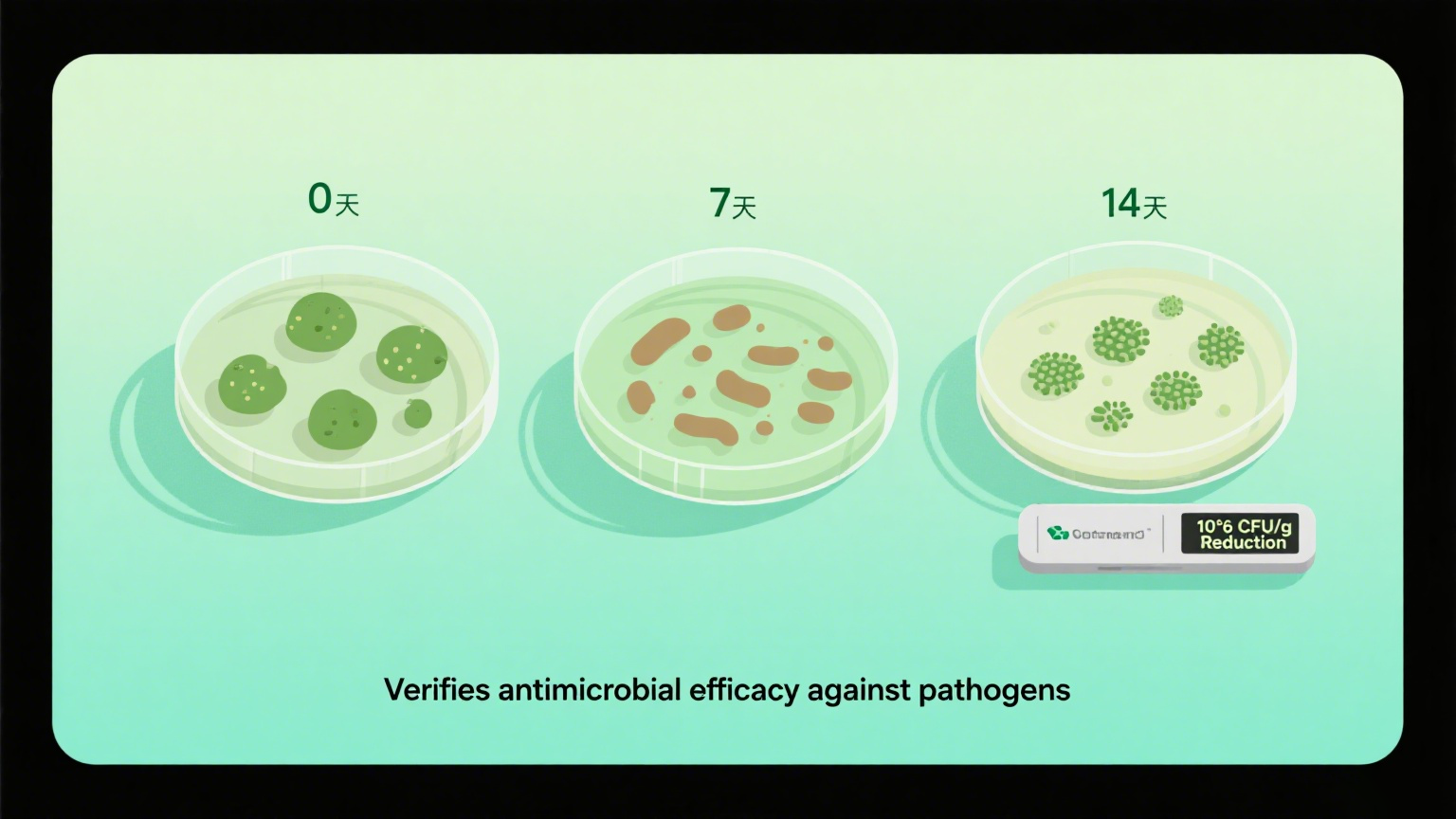
5 Microbial Challenge Test (Preservative Efficacy Test)
Purpose:
To test the effectiveness of the wipes liquid's preservative system in preventing microbial contamination.
Method (performed in lab):
Inoculate the formula with common microbes (e.g., Staphylococcus aureus, E. coli)
Monitor microbial count over time (typically 7, 14, 28 days)
Critical for:
Baby wipes liquid
Medical or sanitizing wipes liquid
Alcohol-free or natural wipes liquid formulas
✅ Summary: Stability Testing = Quality Assurance
Test Purpose Recommended For
High Temp Prevent spoilage in hot conditions Summer or overseas shipping
Freeze– Thaw Prevent separation in cold Export, winter, or cold climates
pH Stability Ensure safety and preservative integrity All wipes liquid types
Centrifuge Check emulsion stability Oil-based or fragranced wipes
Microbial Challenge Validate preservative system Baby, medical, alcohol-free
Conducting these tests before filling not only helps reduce customer complaints and product returns, but also ensures long-term customer trust and brand reputation.




 English
English
 USA
USA
 西班牙语
西班牙语
 俄罗斯
俄罗斯
 葡萄牙
葡萄牙
 印尼
印尼
 巴基斯坦
巴基斯坦
 尼日利亚
尼日利亚
 孟加拉
孟加拉
 墨西哥
墨西哥
 越南
越南
 日本
日本
 韩国
韩国

