
Five Common Causes and Professional Solutions for Wipes Liquid Formulation
Wet wipes are essential in modern life — from household cleaning and baby care to industrial and public disinfection.
Although they appear simple, wet wipes and their wipes liquid are a complex system involving chemistry, microbiology, packaging science, and process control.
During production, storage, and transportation, wet wipes are prone to mold, discoloration, bulging, and odor — problems that can cause product recalls and damage brand reputation.
This article, based on real factory experience, analyzes five common causes behind these issues and offers professional solutions focused on the wipes liquid formulation — the true heart of every wet wipe product.

1️⃣ Mold on Wet Wipes – When the Preservative System Fails
A manufacturer launched a “natural” wipes liquid formula and soon faced customer complaints:
“The wipes are moldy!”
Investigation revealed:
The wipes liquid used only one preservative at too low a concentration.
The pH exceeded the preservative’s effective range.
Natural plant extracts served as nutrients for microorganisms.
The pure water and filling systems were contaminated.
Packaging seals were not tight, allowing secondary contamination.
Solutions:
Design a broad-spectrum, multi-component preservative system for the wipes liquid.
Control pH within the preservative’s optimal range.
Disinfect the pure water system and filling equipment regularly.
Use high-quality, well-sealed packaging.
Conduct preservative challenge tests to ensure long-term stability.
✅ Key takeaway: Mold is rarely caused by one single issue — only when formula, environment, and packaging work together can true microbial safety be achieved.
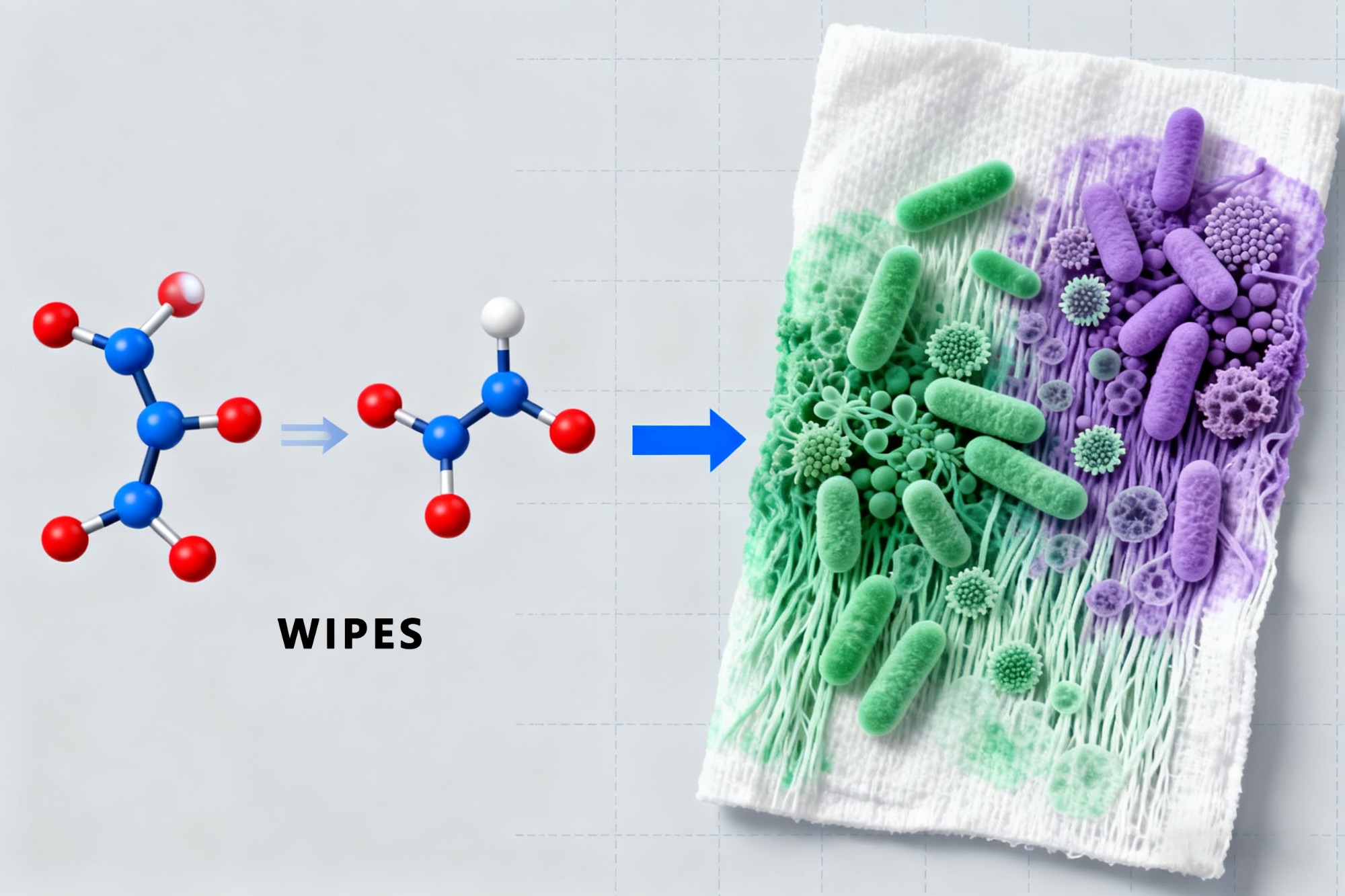
2️⃣ Discoloration – Chemical Reactions or Microbial Activity?
Color changes such as yellowing, browning, or even pink and bluish-green wipes often originate from the wipes liquid chemistry or microbial metabolism.
Common causes:
Oxidation of natural plant extracts in the wipes liquid.
Metal ions reacting with formula ingredients.
pH fluctuations degrading sensitive actives.
Chromophilic bacteria growing due to poor preservation.
Solutions:
Add antioxidants or use nitrogen/inert gas filling.
Use chelating agents to bind metal ions.
Choose stable natural extracts.
Strengthen the preservative system to prevent chromophilic bacteria.
Remember: Discoloration isn’t just cosmetic — it signals chemical or microbiological instability within the wipes liquid.

3️⃣ Bulging Packs – Gas Formation and Packaging Issues
Swollen or inflated wipe packs often indicate microbial gas generation in the wipes liquid.
Possible reasons:
Bacterial growth producing CO₂ and other gases.
Poor barrier performance or leaky heat seals.
Filling at high temperature, causing pressure changes after cooling.
Solutions:
Verify the wipes liquid’s preservative efficacy.
Use multilayer, high-barrier film materials.
Optimize filling temperature and sealing pressure/time.
Store products away from heat and humidity.
Tip: Bulging packs are a warning sign — never ignore them.

4️⃣ Unpleasant Odors – Hidden Chemical and Microbial Sources
Unwanted smells often originate from:
Microbial growth (musty odor).
Incompatible preservatives or surfactants (pungent odor).
Raw material oxidation (fuel-like odor).
Packaging material migration (plastic odor).
Solutions:
Choose low-odor or deodorized raw materials for wipes liquid.
Use stable preservatives and antioxidants.
Control humidity and temperature during production and storage.
Apply safe fragrances or odor absorbers only if necessary.
Rule of thumb: The best way to eliminate odors is to prevent them at the wipes liquid source, not cover them up afterward.

5️⃣ Packaging and Processing – The Final Line of Defense
Even a perfect wipes liquid formula can fail if the packaging process is weak.
Common packaging problems:
High oxygen permeability leading to oxidation or microbial growth.
Low sealing temperature causing air leakage.
Poor flap adhesion.
Inconsistent film batches.
Solutions:
Use high-barrier laminated film.
Strictly control sealing temperature, pressure, and dwell time.
Conduct drop, squeeze, and aging tests.
Audit packaging material suppliers regularly.
Packaging quality often determines the last 10% of wipe stability — don’t underestimate it.
Conclusion – Wet Wipe Stability is a System, Not a Single Step
Mold, discoloration, bulging, and odor are symptoms of system imbalance involving formulation, production, packaging, storage, and logistics.
To build a robust quality control system, wet wipe factories must ensure:
Scientifically designed wipes liquid preservative and antioxidant systems
Regular disinfection of pure water and filling equipment
High-barrier packaging with airtight seals
Temperature and humidity monitoring across the production chain
Only when each link in the system is optimized can wet wipes remain safe, stable, and export-ready.
Professional Wipes Liquid Solutions for Wet Wipe Manufacturers
We specialize in wipes liquid formulations, preservative systems, and functional additive design for B2B wet wipe producers.
Our team helps factories solve quality problems from the root — ensuring long-term product stability, compliance, and customer trust.
Contact us for technical support or customized wipes liquid solutions for your factory.




 English
English
 USA
USA
 西班牙语
西班牙语
 俄罗斯
俄罗斯
 葡萄牙
葡萄牙
 印尼
印尼
 巴基斯坦
巴基斯坦
 尼日利亚
尼日利亚
 孟加拉
孟加拉
 墨西哥
墨西哥
 越南
越南
 日本
日本
 韩国
韩国

